
When a patient takes a pill that combines two medicines-like blood pressure drugs or asthma treatments-it’s not enough to prove the individual parts work. Regulators need to know the whole package delivers the right amount of each drug, at the right speed, every single time. That’s what bioequivalence means for combination products. But testing this isn’t like checking a single-pill generic. The complexity explodes when two active ingredients interact, when a drug is delivered through an inhaler, or when a cream must penetrate skin just right. And right now, the system is struggling to keep up.
Why Bioequivalence Matters for Combination Products
Combination products aren’t just two drugs in one pill. They’re fixed-dose combinations (FDCs), topical creams with dual actives, or drug-device systems like inhalers and auto-injectors. These aren’t rare exceptions-they make up 73% of all new chemical entities approved between 2010 and 2019. The goal of bioequivalence testing is simple: prove a generic version performs just like the brand-name product. No extra clinical trials. No patient risk. Just science-based proof that the body absorbs the same amount of each active ingredient, at the same rate.
But here’s the catch: if you’re testing a generic version of a combination product, you can’t just compare it to the brand’s combo. You also have to prove it matches the individual drugs taken separately. That means designing studies where volunteers take four different regimens: the brand combo, the generic combo, the brand’s individual drugs taken together, and the generic’s individual drugs. It’s expensive. It’s time-consuming. And it’s not always clear how to do it right.
The Three Big Categories of Challenge
Not all combination products are the same. Each type-FDCs, topical products, and drug-device systems-has its own unique bioequivalence headaches.
Fixed-Dose Combinations (FDCs) are the most common. Think of HIV meds like dolutegravir/lamivudine or diabetes drugs like sitagliptin/metformin. The problem? The two drugs can interfere with each other. One might change how the other dissolves, or affect how fast it’s absorbed. The FDA now requires FDC generics to show bioequivalence to both the combo and the individual components. That means three-way crossover studies with 40 to 60 healthy volunteers-twice as many as a standard single-drug study. And even then, 25-30% of these studies fail because of unexpected interactions between the ingredients.
Topical products like creams, ointments, and foams are even harder. You can’t just measure drug levels in blood. The drug has to reach the right layer of skin-the stratum corneum-to work. The FDA says you need to use tape-stripping: peeling off 15-20 layers of skin and measuring how much drug is in each. But there’s no standard on how thick each layer should be, how much drug to extract, or how to compare results across labs. One company spent three years trying to get a generic calcipotriene/betamethasone foam approved. All three bioequivalence studies failed-not because the product didn’t work, but because the measurements were inconsistent between tests.
Drug-device combinations-like inhalers, nasal sprays, or auto-injectors-are a whole other level. The device isn’t just packaging. It’s part of the drug delivery system. If the inhaler’s nozzle is slightly smaller, or the injector’s spring tension is off by 5%, the dose delivered can change dramatically. The FDA requires aerosol particle size to be within 80-120% of the brand product. But measuring that requires expensive equipment, trained technicians, and controlled environments. And here’s the kicker: 65% of complete response letters from the FDA for generic inhalers cite problems with user interface testing. That means even if the drug is perfect, if the device feels different to the patient, it gets rejected.

Why Standard Bioequivalence Rules Don’t Work
For a single oral drug, the rules are simple: measure the peak concentration (Cmax) and total exposure (AUC) in 24-36 volunteers. If the generic’s values fall between 80% and 125% of the brand’s, it’s approved. That’s the gold standard.
But for combination products, those numbers don’t tell the whole story. FDCs often need tighter limits-like 90-111%-for drugs with narrow therapeutic windows. Modified-release versions, like slow-dissolving tablets, fail 35-40% of the time in initial submissions. And for topical products, clinical endpoint studies (where you actually measure skin improvement in patients) can cost $5-10 million and take years. That’s not feasible for most generic manufacturers.
There’s also inconsistency in how regulators interpret the rules. One FDA division might accept a certain method for measuring drug penetration, while another rejects it. Between 2021 and 2023, 78 industry submissions to the FDA’s public docket called out ‘lack of clear bioequivalence pathways’ as the top barrier. Small companies, especially, get stuck. They can’t afford the consultants, the specialized labs, or the repeated trial runs.
What’s Being Done to Fix It
The system isn’t broken-it’s just outdated. And change is coming, slowly.
The FDA launched its Complex Generic Products Initiative in 2018 and has since created the Complex Product Consortium. This group has developed 12 product-specific bioequivalence recommendations. Companies that follow them see development timelines shrink by 8-12 months. The FDA’s 2024 draft guidance includes 15 new recommendations, including one for HIV FDCs that now requires simultaneous bioequivalence testing of both drugs.
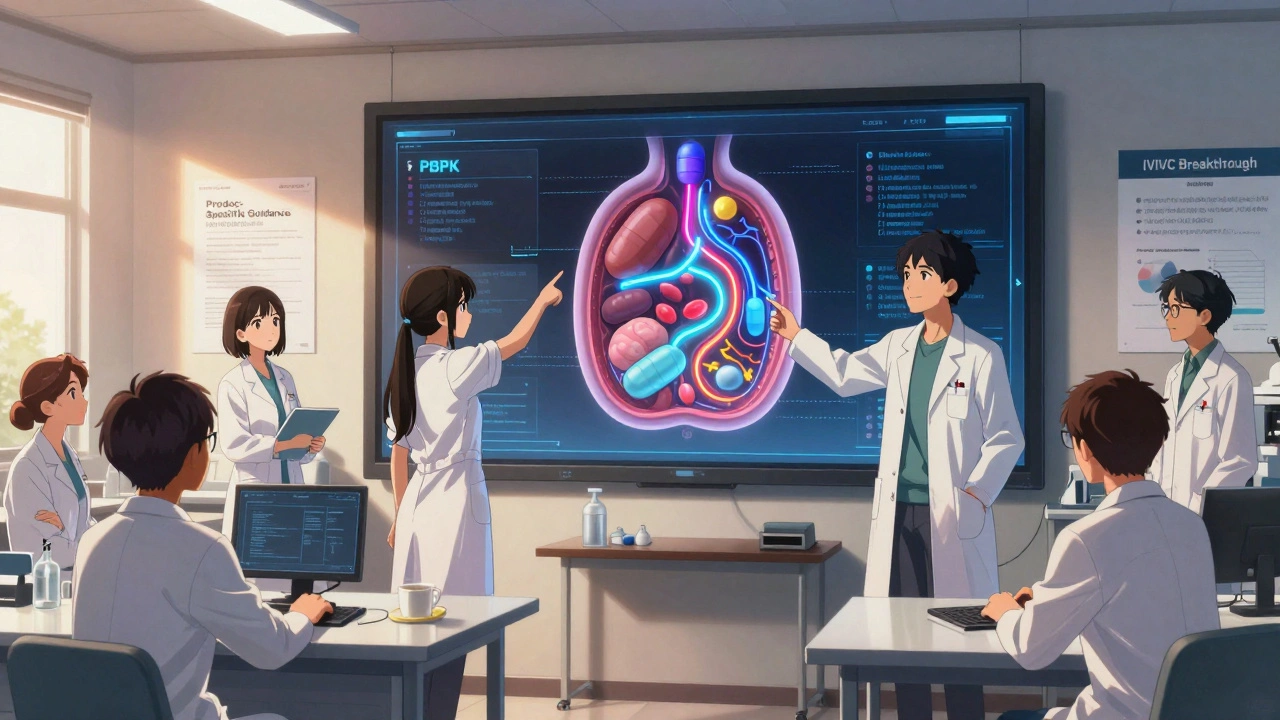
One major breakthrough is the use of physiologically-based pharmacokinetic (PBPK) modeling. Instead of running dozens of human trials, companies can simulate how the drug behaves in the body using computer models. Seventeen ANDAs for complex products have already been approved using PBPK modeling, cutting clinical study needs by 30-50%. That’s huge for cost and speed.
Another promising area is in vitro-in vivo correlation (IVIVC) for topical products. Researchers are finding that if you can accurately measure drug release and penetration in the lab using tape-stripping, you can predict how it will behave in real patients-with up to 85% accuracy. If this becomes standard, it could eliminate the need for massive patient trials.
The FDA is also working with NIST to create reference standards-physical samples that labs can use to calibrate their equipment. For inhalers, those standards are expected by late 2024. This will reduce variability between labs and make results more reliable.
The Real Cost of Delay
Behind every delay in approval is a patient waiting for an affordable option. Generic drugs saved the U.S. healthcare system $373 billion in 2020 alone. But complex products? They’re the slowest to come to market. While standard generics get approved in 14.5 months on average, combination products take 38.2 months. That’s over two extra years of patients paying brand prices.
And it’s not just about money. In the U.S., 312 complex products are on the FDA’s watchlist. Topical products make up 38%, inhalers 22%, and FDCs 19%. If bioequivalence challenges aren’t solved, 45% of these brand products may have no generic competition by 2030. That means millions of patients stuck with high-cost treatments.
For companies, the stakes are high too. Teva reported that 42% of their complex product failures were due to bioequivalence issues. Mylan (now Viatris) saw development timelines stretch by 18-24 months for topical products. And litigation over patents for combination products has tripled since 2019, adding more delays.
What’s Next?
The future of bioequivalence for combination products isn’t about doing the same tests harder. It’s about doing smarter tests.
Regulators are moving toward product-specific guidance instead of one-size-fits-all rules. That’s good. But it needs to happen faster. The FDA’s goal of creating 50 new product-specific guidances by 2027 is ambitious-and necessary.
Companies need to invest in modeling, advanced analytics, and standardized testing methods. Labs need better reference materials. And regulators need to be more transparent about what they’re looking for.
At the end of the day, bioequivalence isn’t just a technical hurdle. It’s a bridge between innovation and access. If we can solve these testing challenges, we unlock cheaper, more available treatments for chronic diseases-from asthma to HIV to eczema. That’s not just good science. It’s good health policy.
What is bioequivalence for combination products?
Bioequivalence for combination products means proving that a generic version delivers the same amount of each active ingredient, at the same rate, as the brand-name product. This applies to fixed-dose combinations (like two pills in one), topical creams with dual drugs, or drug-device systems like inhalers. The goal is to ensure therapeutic equivalence without requiring new clinical trials.
Why are combination products harder to test than single-drug generics?
Combination products involve multiple active ingredients or delivery mechanisms that can interact. One drug might affect how another is absorbed. Topical products need to penetrate skin layers precisely, and drug-device systems require matching not just the drug but also the device’s performance-like inhaler spray patterns or injector force. These complexities demand larger studies, specialized equipment, and often, new testing methods that aren’t standardized yet.
What’s the biggest hurdle for generic inhalers?
The biggest hurdle is comparative user interface assessment. Even if the drug dose is identical, if the inhaler feels different-harder to press, different spray pattern, or different mouthpiece design-patients may use it incorrectly. The FDA rejects 65% of generic inhaler applications due to deficiencies in this area. Testing requires measuring aerosol particle size, drug deposition, and patient handling-all under controlled conditions.
How long does it take to get a combination product approved as a generic?
On average, it takes 38.2 months for a combination product to get first-cycle approval from the FDA-more than double the 14.5 months for standard single-drug generics. Delays come from complex study designs, inconsistent regulatory feedback, and the need for multiple rounds of testing due to failed bioequivalence results.
Are there any new technologies helping with bioequivalence testing?
Yes. Physiologically-based pharmacokinetic (PBPK) modeling lets companies simulate how drugs behave in the body using computer models, reducing the need for human trials. In vitro-in vivo correlation (IVIVC) for topical products shows that lab-based skin measurements can predict real-world performance with up to 85% accuracy. The FDA is also working with NIST to create reference standards for inhalers and other devices to improve testing consistency across labs.
Why do some bioequivalence studies fail even when the product works?
Studies often fail because of variability in testing methods-not because the product is ineffective. For topical products, differences in tape-stripping depth or drug extraction techniques can lead to inconsistent results. For inhalers, slight changes in device manufacturing affect aerosol delivery. Even small differences in how volunteers take the drug-like timing or food intake-can skew results in multi-drug studies. The problem isn’t the drug; it’s the lack of standardized, reliable testing protocols.




Comments (15)
sagar bhute
The entire regulatory framework for combination products is a joke. They demand triple the testing, triple the cost, and still can't agree on what 'bioequivalence' even means. It's not science-it's bureaucratic theater. Companies spend millions and get rejected for a 3% variance in spray pattern. Meanwhile, patients die waiting for affordable meds. Fix the system or get out of the way.
Cindy Lopez
There are multiple grammatical inconsistencies in the original post, particularly around the use of hyphens in compound modifiers. For example, 'drug-device systems' should always be hyphenated when used attributively, but in several instances it is not. This undermines the credibility of an otherwise well-researched article.
James Kerr
Hey, this is actually super interesting. I never realized how wild it is that an inhaler’s nozzle being 5% off can get a whole generic drug rejected. 🤯 It’s not just about the medicine-it’s about how you hold the thing. Kinda makes you wonder if we’re testing drugs or user manuals at this point. Glad to see they’re using computer models now though. That’s the future.
shalini vaishnav
India has been producing high-quality generic drugs for decades without this nonsense. Why does the FDA demand such absurd, over-engineered protocols? We’ve been exporting billions in affordable FDCs to Africa and Southeast Asia using simple, proven methods. This American over-regulation is just protectionism disguised as science. The world doesn’t need 15 layers of tape-stripping to know if a cream works.
bobby chandra
Let me tell you something-this whole system is rigged. Big Pharma writes the rules, small companies get crushed, and patients pay the price. PBPK modeling? IVIVC? These aren’t breakthroughs-they’re survival tools for companies that can afford $20 million in R&D. Meanwhile, the FDA sits on their hands for three years while a kid with asthma can’t breathe because his inhaler costs $500. We’re not fixing a broken system-we’re just making the rich richer.
Archie singh
Regulators don’t care about patients. They care about liability. If a generic inhaler fails and someone dies, they get sued. So they demand impossible precision on device interfaces, even if the drug is identical. The real problem? No one’s held accountable for brand-name products that fail in the real world. But god forbid a generic has a slightly different button click.
Albert Essel
I appreciate how nuanced this breakdown is. The tension between scientific rigor and accessibility is real, and it’s not a matter of being right or wrong-it’s about finding balance. PBPK modeling and reference standards are steps in the right direction. But we also need more transparency from regulators and less fear-driven decision-making. Patients aren’t test subjects. They’re people waiting for relief.
Charles Moore
One thing that’s often missed: the human factor. Even if a generic inhaler delivers the exact same dose, if the patient feels it’s harder to use, they won’t take it properly. That’s not a failure of science-it’s a failure of design thinking. We need more input from actual patients and caregivers in the testing process. Not just lab technicians.
Gavin Boyne
So let me get this straight. We’ve got a system where you can’t prove a drug works unless you prove the patient can hold the device the same way as the brand version… but we don’t even test whether the brand version actually works consistently in real life? Brilliant. Pure genius. The FDA’s version of a Turing test: if the patient can’t tell the difference, it’s bioequivalent. Except we’re not testing the patient-we’re testing their dexterity.
Rashi Taliyan
I had a cousin who couldn’t afford his asthma inhaler for two years. He used the same one until the plastic cracked. He’d shake it like a soda can just to get one puff. When he finally got a generic, he cried because it felt different. He didn’t care about the science-he just wanted to breathe. This isn’t about data points. It’s about dignity.
Kara Bysterbusch
It is imperative to acknowledge the profound implications of regulatory fragmentation across international jurisdictions. The FDA’s stringent criteria, while scientifically rigorous, create a significant barrier to global market access for generic manufacturers in low- and middle-income countries. Harmonization of bioequivalence standards through WHO and ICH frameworks is not merely desirable-it is ethically obligatory.
Rashmin Patel
Guys, I’ve been working in a lab that does tape-stripping for topical generics, and let me tell you-it’s a nightmare. One lab uses 15 layers, another uses 20. One uses acetone extraction, another uses saline. The results are all over the place. We spent six months trying to replicate our own data. And now the FDA wants us to prove bioequivalence? We can’t even prove consistency within our own building. 😭 We need one standard. One. Please. Someone just pick a method and stick with it.
vinoth kumar
Big pharma doesn’t want generics to win. They fund the FDA’s guidelines. They lobby for complex testing because it keeps cheaper drugs off the market. It’s not about safety-it’s about profit. Look at the timeline: 14 months for single drugs, 38 for combos. That’s not science. That’s a business model.
Gene Linetsky
Did you know the FDA’s reference standards for inhalers were designed by a contractor who used to work for GlaxoSmithKline? Coincidence? I don’t think so. PBPK modeling? That’s just a fancy way of saying ‘we trust the math more than the data.’ And the math is written by the same companies that own the patents. This isn’t regulation-it’s a rigged casino.
Ignacio Pacheco
So the FDA rejects a generic because the inhaler clicks differently… but the brand version’s click has been changing every year since 2015? That’s the real issue. The brand isn’t consistent. Why are we holding generics to a moving target?