
When you pick up a generic prescription at the pharmacy, you might assume it’s just a cheaper version of the brand-name drug. But here’s the truth: generic drugs don’t just match the price-they have to match the performance, down to the last milligram. The U.S. Food and Drug Administration (FDA) doesn’t approve generics because they’re affordable. They approve them because they’re scientifically identical in safety, strength, and quality to the original. And that’s not a guess. It’s a requirement backed by hard data, strict testing, and years of oversight.
How the FDA Ensures Generic Drugs Work the Same Way
The legal backbone of generic drug approval is the Hatch-Waxman Act of 1984. It created the Abbreviated New Drug Application (ANDA) pathway-a faster, smarter way to bring generics to market without repeating every clinical trial ever done on the brand-name drug. Instead of starting from scratch, generic manufacturers prove their product behaves the same way in the body. That’s called bioequivalence.
Here’s how it works: A generic drug must deliver the same active ingredient, in the same amount, at the same rate, and through the same route as the brand-name version. If you take a 20 mg tablet of the brand-name drug, the generic must also be a 20 mg tablet, taken orally, and it must release that 20 mg into your bloodstream at the same speed and to the same extent.
The gold standard test? Blood concentration studies. Healthy volunteers take both the brand-name and generic versions in separate sessions, and researchers measure how much of the drug enters the bloodstream over time. The key numbers are Cmax (peak concentration) and AUC (total exposure). For approval, the generic’s results must fall between 80% and 125% of the brand’s. That’s not a wide margin-it’s a tight band designed to ensure no meaningful difference in how the drug works.
Strength, Purity, and Stability: What the FDA Checks
It’s not enough for a generic to work the same in your blood. It has to be made the same way, every single time.
The FDA requires generic manufacturers to prove their product meets exact standards for:
- Strength: Each tablet or capsule must contain the labeled amount of active ingredient, within a very narrow tolerance.
- Purity: No harmful impurities. Even trace contaminants must be below FDA-set limits.
- Stability: The drug must remain effective and safe throughout its shelf life, even under heat, humidity, or light exposure.
This isn’t theoretical. Every batch of a generic drug is tested for these qualities before it leaves the factory. The FDA doesn’t trust paperwork alone-they inspect the facilities. In 2022, the agency conducted over 1,200 pre-approval inspections of manufacturing sites worldwide. If a plant fails a single inspection for poor sanitation, inconsistent mixing, or faulty equipment, the application is put on hold until the issue is fixed and re-inspected.
One manufacturer learned this the hard way in 2021. The FDA rejected a generic version of Jardiance because tablet hardness varied across batches. That might sound minor-but inconsistent hardness can mean inconsistent dissolution, which means inconsistent absorption. And that’s a safety risk.
Why Some Generics Take Years to Get Approved
Not all generics are created equal. Simple pills-like metformin or lisinopril-are approved quickly. But complex products? That’s a different story.
Inhalers, injectables, topical creams, and extended-release tablets are harder to copy. Why? Because they rely on more than just chemistry. Their delivery systems matter. An inhaler isn’t just a chemical-it’s a pressurized canister with a precise valve. A long-acting pill isn’t just a tablet-it’s a tiny time-release machine inside.
For these complex generics, the FDA demands more. Take Ritalin LA, an extended-release methylphenidate. The generic must match not just the total absorption but the pattern of release: how much drug is released in the first 3 hours, next 4 hours, and final 5 hours. That requires multiple blood draws and complex modeling. Only 58% of complex generic applications get approved within three tries, compared to 76% for simple pills.
Even after approval, delays can happen. Between 2015 and 2020, the FDA approved only 3 out of 27 applications for generic EpiPens-not because the drug didn’t work, but because the auto-injector device was too hard to replicate. The needle mechanism, the spring tension, the click sound-all had to be identical to ensure patients could use it correctly in an emergency.
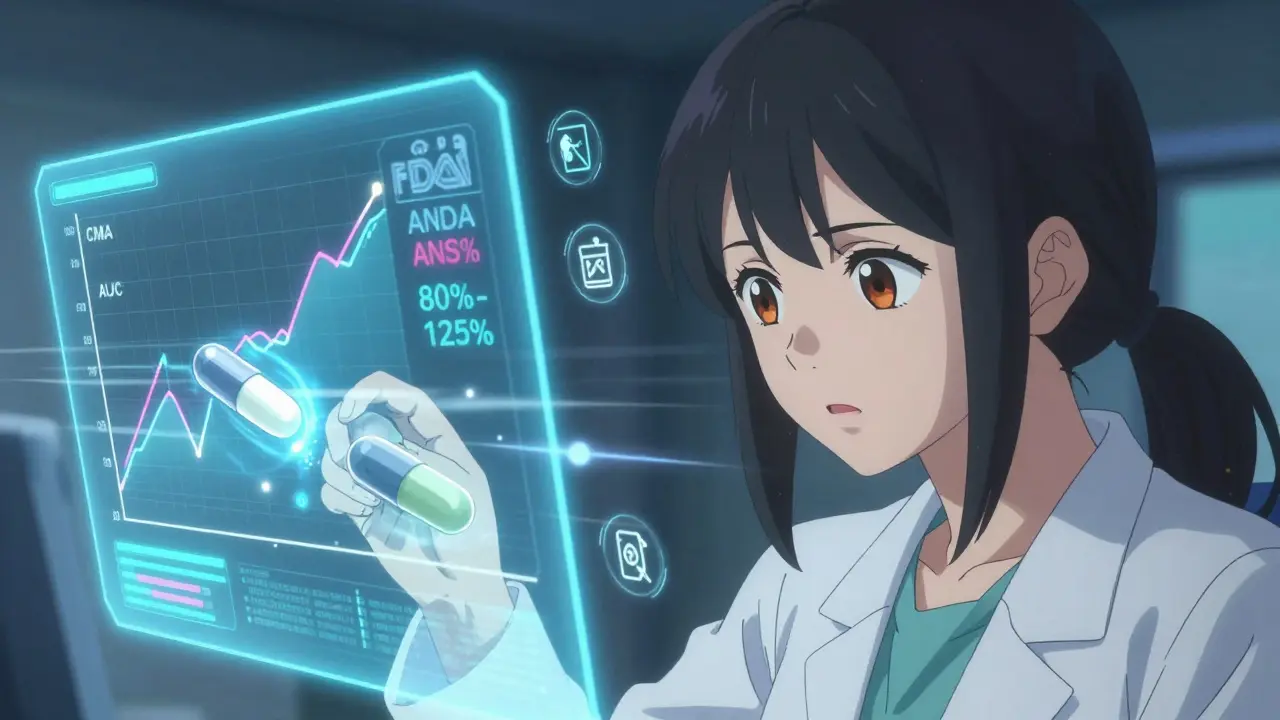
Narrow Therapeutic Index Drugs: When Even Small Differences Matter
Some drugs have a razor-thin line between working and causing harm. Warfarin (a blood thinner), levothyroxine (for thyroid conditions), and phenytoin (for seizures) are in this category. A 10% change in blood levels can mean the difference between preventing a clot and causing a stroke-or between controlling seizures and triggering them.
For these, the FDA doesn’t use the standard 80%-125% range. It tightens the rules. For generic levothyroxine, the requirement is now 95%-105%. For some, it’s even tighter. This isn’t just policy-it’s science. Studies show patients switching between different brands of levothyroxine can experience changes in thyroid hormone levels that require dose adjustments. The FDA’s stricter limits prevent that.
Despite this, some experts still push for even tighter standards. Dr. Randall Uppal, in a 2022 journal article, argued that even 95%-105% might be too loose for patients on multiple interacting drugs. The FDA continues to evaluate these cases individually, and the trend is clear: stricter rules for high-risk drugs.
Who Makes These Drugs-and Why It Matters
Most people assume generic drugs are made in the U.S. But the reality is more global. About 80% of the active ingredients in U.S. generics come from India and China. Final manufacturing happens in facilities from New Jersey to Hyderabad.
The FDA inspects all of them-same rules, same standards. But the logistics are harder. A single ANDA submission can include 5,000 to 10,000 pages of data: chemistry reports, stability studies, manufacturing protocols, bioequivalence results, and labeling comparisons. The average cost to develop a simple generic? $1.3 million. For a complex one? Up to $25 million.
And it takes time. The average approval timeline is 32.7 months. For complex drugs? Nearly four years. That’s why only 107 generic drugs were approved in 2022, and 90 in 2023-despite thousands of applications being filed. The bottleneck isn’t the science. It’s the paperwork, the inspections, and the complexity.

What Happens After Approval?
Approval isn’t the finish line. The FDA keeps watching. Every generic drug is monitored through post-market surveillance. The agency tracks adverse events, recalls, and manufacturing issues long after the drug hits shelves.
And the data supports the system. A 2021 report by the American Medical Association found that for 98.7% of therapeutic categories, generics performed identically to brand-name drugs in real-world use over 15 years. That’s not luck. That’s regulation.
Patients get the same effectiveness. Insurers pay less. The healthcare system saves billions. In 2022, generics saved U.S. patients $373 billion. That’s not a marketing slogan-it’s a number from IQVIA’s global drug use report.
What’s Next for Generic Drugs?
The FDA’s 2023 GDUFA III plan aims to speed up approval of complex generics. By 2027, they want to approve half of these harder-to-copy drugs within two review cycles-up from just 28% today. That means more generics for expensive drugs like Humira, EpiPen, and Vivitrol.
But challenges remain. Patent thickets, legal delays, and evergreening tactics still block generics from entering the market-even after FDA approval. In 2023, the FTC found that on average, generics wait 2.4 years after approval before they can actually be sold.
Still, the system works. It’s not perfect. But for the 90% of prescriptions filled with generics in the U.S., it’s the reason they’re safe, effective, and affordable.
Are generic drugs as safe as brand-name drugs?
Yes. The FDA requires generic drugs to meet the same safety standards as brand-name drugs. Every generic must contain the same active ingredient, in the same strength, and deliver the same therapeutic effect. Post-market surveillance shows no significant difference in adverse events between generics and brand-name drugs across 98.7% of therapeutic categories.
Why do some generic drugs look different from the brand name?
U.S. law prohibits generics from looking exactly like brand-name drugs, so they may differ in color, shape, or markings. But these differences are only cosmetic. The active ingredient, dosage, strength, and effectiveness are identical. The FDA ensures that appearance changes don’t affect how the drug works.
Do generic drugs take longer to work than brand-name drugs?
No. Bioequivalence testing requires that generic drugs reach the same peak concentration in the blood at the same rate as the brand-name version. For immediate-release pills, this means the same onset time. For extended-release versions, the release pattern must match exactly, ensuring the same duration and timing of effect.
What’s the difference between a generic and a biosimilar?
Generics are exact copies of small-molecule drugs made from chemicals. Biosimilars are highly similar versions of large, complex biologic drugs made from living cells. Biosimilars require more clinical testing because their structure is harder to replicate exactly. The FDA treats them under separate approval pathways.
Can I trust a generic drug made overseas?
Yes. The FDA inspects all manufacturing facilities-whether in the U.S., India, China, or elsewhere-using the same strict standards. Over 1,200 inspections are conducted annually, and no facility is exempt. A drug approved by the FDA is approved regardless of where it’s made.
Why do some doctors still prefer brand-name drugs?
Some doctors may be unfamiliar with newer generics or have had rare patient experiences with switching. But for most medications, clinical evidence shows no difference. For narrow therapeutic index drugs like warfarin or levothyroxine, the FDA has tighter standards, and switching is generally safe when monitored. Patient-specific concerns should be discussed with a provider, but blanket avoidance of generics isn’t supported by data.




Comments (10)
Paul Barnes
Let’s be real-the FDA’s bioequivalence range of 80%-125% is a joke. If your drug’s peak concentration varies by 45% from the brand, that’s not ‘identical,’ that’s a crapshoot. I’ve seen patients on warfarin go from stable INRs to emergency rooms after switching generics. The data says 98.7% are fine, but the 1.3%? They’re the ones who end up dead. And no one talks about that.
Andy Thompson
80% TO 125%?! 😱 That’s not science-that’s corporate bribery! The FDA’s in bed with Big Pharma and Indian factories. You think they inspect those plants? Nah. They get a tour, some free chai, and a ‘certified’ report. I’ve got a cousin in Hyderabad-he says they mix batches with scoops and hope. And you’re telling me this is safe for my kid’s ADHD med?! 🤡🇺🇸
sagar sanadi
USA says generic same. But in India, we make it for $0.02 per pill. You think they care about 80-125%? They care about how many pills fit in one box. FDA? They get paid to say yes. I saw a batch of metformin with sugar instead of medicine. No one got sick. Lucky, right?
kumar kc
If you can’t afford the brand, you shouldn’t be sick. Medicine isn’t a right-it’s a privilege earned by responsibility.
Thomas Varner
Okay, so… the FDA does inspections, right? And they’ve got over 1,200 of them a year? That’s… a lot. And yet, the approval time is still over two years? Huh. So they’re doing a lot of work… but it’s also super slow? Like, why? Is it because there’s too much paperwork? Or because they’re just… cautious? I mean, I get it, safety first, but… wow. It’s kind of wild that a pill that costs $0.10 can take 32 months to get approved…
Art Gar
It is incumbent upon the regulatory apparatus to maintain the highest standard of pharmaceutical integrity. The statistical assurances provided, while statistically significant, do not constitute empirical certainty for every individual patient. The variance thresholds, though mathematically defensible, remain ethically precarious when applied to pharmacologically sensitive populations. One must question whether cost-efficiency has supplanted clinical precision in the current paradigm.
Edith Brederode
I love that the FDA actually inspects factories worldwide 😊 It makes me feel so much safer knowing someone is checking every batch! Also, the fact that generics saved $373 billion?! That’s insane!! 🙌 My mom takes levothyroxine and switched to generic years ago-no issues at all. So proud of how this system works 💖
Arlene Mathison
Guys, if you’re still scared of generics, you’re letting fear run your health. I used to be like that-until I switched my blood pressure med to generic and saved $200 a month. No side effects, no weirdness. The science is solid. Stop overthinking it. Your wallet and your doctor will thank you. 💪💊
Emily Leigh
So… we’re told generics are ‘identical’… but then they’re not allowed to look the same? And the FDA tightens the range for some drugs but not others? And we’re supposed to trust a system where 80% of the active ingredients come from countries we’ve been told to distrust? Hmm. Isn’t that just… cognitive dissonance on a molecular level? 🤔 Maybe the real question isn’t ‘are generics safe?’-but ‘why do we still believe in systems that contradict themselves?’
thomas wall
One must observe with sober clarity that the very notion of bioequivalence, as presently defined, is an elegant fiction. The human body is not a test tube. The pharmacokinetic curves of two compounds, even if statistically indistinguishable, may elicit divergent responses in individuals with comorbidities, genetic polymorphisms, or altered hepatic metabolism. To assert uniform safety is not scientific-it is hubris. The FDA, though diligent, operates within a framework designed for mass utility, not individual nuance. The patient, in this calculus, becomes a statistic.